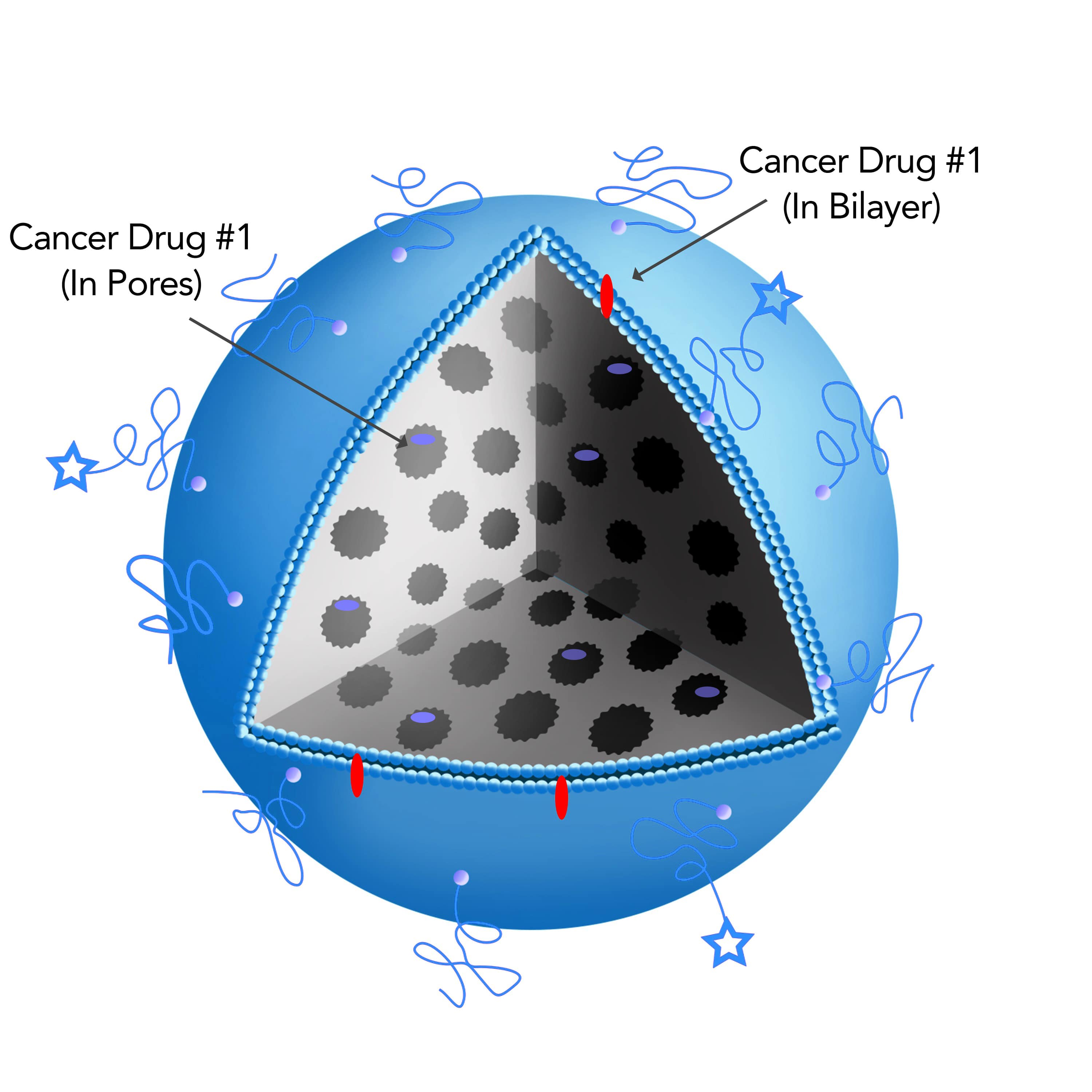
The composition of the Silicasome allows for different drug loading methods that can use both the interior packaging space of the particle as well as its lipid bilayer to incorporate the drugs.
For instance, the irinotecan carrier encapsulates the drugs into the porous interior of the particle, while the combined delivery of gemcitabine and paclitaxel by the silicasome is achieved by loading gemcitabine into the particle pores while incorporating paclitaxel into the lipid bilayer.
It is also possible to put a proton-releasing trapping agent into the porous interior, which could allow multiple weak basic drugs to be remotely imported across the lipid bilayer for drug delivery.